How do you figure out the molar mass or GFW of each compound? |
||
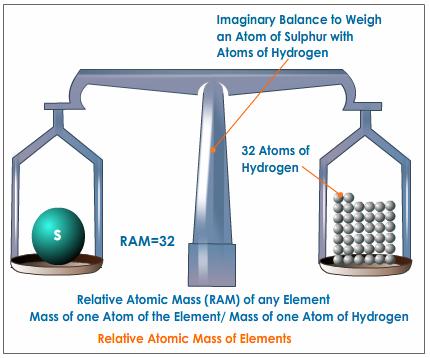
Steps to finding the GFW Before you know how to solve limiting reagents problems, it is important that you know how to find the molar mass (GFW) of a given compound. You can round all atomic masses to a whole number, except for Chlorine's, which stays and is completely rounded to 35.5 g/mol. Here are two example problems to help you get adjusted: 1. C4H10S and O2 Multiply the molar mass of each atom by the number of compounds it contains (ex. multiply carbon's atomic mass by 4 or hydrogens atomic mass by 10) C = 12 (x 4) H = 1 (x 10) S = 32 (x 1) GFW of C4H10S = 90 g/mol O = 16 (x 2) GFW of O2 = 32 g/mol So, the GFW of C4H10S is 90 g/mol, while the GFW of O2 is 32 g/mol. 2. CH4 and Cl2 C = 12 (x 1) H = 1 (x 4) GFW of CH4 = 16 g/mol Cl = 35.5 (x 2) GFW of Cl2 = 72 g/mol So, the GFW of CH4 is 16 g/mol and the GFW of Cl2 is 72 g/mol. |
 |
|